
When you leave a steel bike outside in the rain, you’ll notice orange flakes—classic rust. But what if your bike is made of aluminum? Can aluminum rust in the same way? This is a common question for homeowners, engineers, and anyone using aluminum in everyday life. The answer isn’t as simple as yes or no, and understanding why is the first step to protecting your investment.
Let’s clear up the confusion: aluminum does not rust in the traditional sense. Rust is a term reserved for iron and steel—metals that contain iron. When iron reacts with water and oxygen, it forms iron oxide, flaking away and exposing fresh metal to repeat the cycle. Aluminum, on the other hand, doesn’t contain iron, so it can’t produce true rust. But that doesn’t mean it’s immune to damage.
Instead, aluminum undergoes a different chemical process called oxidation. When exposed to air or moisture, aluminum reacts quickly, forming a thin, hard layer of aluminum oxide on its surface. Unlike rust, this layer is usually white or dull gray, and—here’s the fascinating part—it’s often protective. The oxide layer acts as a natural shield, preventing further aluminum corrosion in most environments. Imagine it like a self-healing skin: if the surface is scratched, the exposed aluminum instantly forms a new protective layer.
However, this protection isn’t foolproof. In certain conditions—like exposure to saltwater, industrial chemicals, or when the oxide layer is repeatedly damaged—aluminum corrosion can become a serious concern. That’s why it’s important to understand not just if aluminum can rust, but how and why it corrodes, and what steps you can take to keep it strong and beautiful for years to come.
In this guide, we've broken down the fundamental science behind aluminum corrosion and how it differs from rust. For a deeper dive into the specific mechanisms of aluminum's unique oxidation, the factors that accelerate corrosion in different environments, and detailed strategies for protecting your aluminum products—whether it's outdoor furniture, marine components, or architectural elements—explore our comprehensive blog post: Can Aluminum Rust? Unpacking the Science of Its Unique Corrosion.
Whether you’re a DIY enthusiast or a professional in the field, understanding these principles is key to maximizing the longevity and performance of your aluminum investments.
When you hear someone ask, "Does aluminum rust?" it's easy to assume the answer is a simple yes or no. But the truth is a bit more nuanced—and understanding the difference between rust and corrosion is key to protecting your aluminum items.
So, can aluminum rust? The answer is no—aluminum cannot rust because it contains no iron. However, aluminum does corrode when exposed to air and moisture. Instead of rusting, it forms a thin layer of aluminum oxide. This layer is typically white or dull gray and, unlike rust on iron, it usually protects the underlying metal from further damage rather than causing it to flake away.
In summary, when people talk about "aluminum rust," what they’re really asking is if aluminum can corrode. The answer is yes—but the process is different, and often less destructive, than the rusting you see on iron or steel. Next, let’s explore the science behind these differences and why they matter for your projects.

Ever wondered why an old iron gate turns orange and flaky, while your aluminum patio furniture just looks a bit dull? The answer lies in the unique ways these metals react with their environment. Let’s break down the science and the visuals behind aluminum oxidation and classic iron rust, so you’ll know exactly what to expect—and why aluminum still needs protection in certain settings.
Sounds complex? Here’s a simple way to look at it: both iron and aluminum react with oxygen and water, but the results are very different. Iron forms rust (iron oxide), which is porous and flakes away, exposing more metal to damage. Aluminum, on the other hand, forms a thin, hard layer of aluminum oxide—usually invisible or chalky white—that actually helps shield the metal underneath.
| Aspect | Iron (Rust) | Aluminum (Oxidation) |
|---|---|---|
| Chemical Reaction | Iron + Oxygen + Water → Iron Oxide (Rust) |
Aluminum + Oxygen → Aluminum Oxide Aluminum + Water → Aluminum Hydroxide (in some conditions) |
| Appearance | Orange-brown, flaky, and rough surface | Dull gray or white; thin, hard, often invisible or slightly chalky layer |
| Protective Qualities | Rust is porous and weak—keeps flaking off, exposing fresh metal to more corrosion | Aluminum oxide is dense and adherent—creates a barrier that slows further corrosion |
| Speed of Formation | Can start within hours in the presence of water and oxygen | Forms almost instantly when aluminum is exposed to air or moisture |
| Long-Term Effect | Progressive weakening and eventual structural failure | Usually protects the metal, but can be compromised in harsh environments (like saltwater or acidic conditions) |
In summary, while aluminum doesn’t rust like iron, aluminum oxidation is a double-edged sword: it’s mostly protective, but not invincible. Up next, we’ll explore how different types of aluminum—pure, alloyed, cast, or anodized—respond to corrosion and what that means for their durability in real-world applications.
Ever wondered why some aluminum products seem to last forever, while others show signs of damage much sooner? The answer lies in the type of aluminum and the treatments it receives. Not all aluminum is created equal—each variety offers unique levels of corrosion resistance, and understanding these differences can help you choose the right material for your needs.
Let’s break it down with real-world examples and simple comparisons. When you’re asking, “Can cast aluminum rust?” or “Can anodized aluminum rust?,” you’re really asking how these different forms respond to environmental stress. Here’s what you need to know:
Beyond anodizing, other surface treatments like powder coating and wet painting further enhance corrosion protection. These finishes add extra layers that seal the aluminum from environmental threats, while also allowing for a wide range of colors and textures.
Imagine you’re choosing aluminum window frames for a coastal home or structural profiles for a new rail project. The right combination of alloy and surface treatment can mean the difference between decades of maintenance-free performance and premature failure.
For demanding applications—whether it’s public transportation, high-rise buildings, or energy-efficient window systems—partnering with an expert manufacturer is crucial. Shengxin Aluminum specializes in advanced surface treatments, including anodizing and powder coating, to produce highly durable, corrosion-resistant aluminum profiles. Their expertise ensures that each profile not only meets strength and appearance standards but also stands up to harsh environments for years to come.
Next, let’s see how water and moisture specifically impact aluminum’s long-term durability, including what happens when the protective layer is challenged.

Imagine leaving an aluminum ladder outside after a rainstorm or noticing condensation on your aluminum window frames. You might wonder: Can aluminum rust in water? While aluminum doesn’t rust like iron, water exposure still plays a significant role in its long-term durability.
Aluminum’s first line of defense is its thin oxide layer, which forms instantly when the metal is exposed to air. This layer acts like armor, shielding the underlying metal from further attack. But what happens when water enters the picture?
Even if you’re not dunking aluminum directly in water, humidity in the air can still contribute to corrosion. Here’s how:
So, while aluminum is generally resilient, aluminum corrosion in water becomes a concern if the protective oxide layer is breached, if water is contaminated, or if the surface stays wet for long periods. You might see white spots, powdery deposits, or small pits—subtle signs that the metal’s defense is being challenged.
Next, let’s explore why saltwater and contact with other metals can pose even greater threats to aluminum’s longevity—and what you can do to prevent these specific types of corrosion.
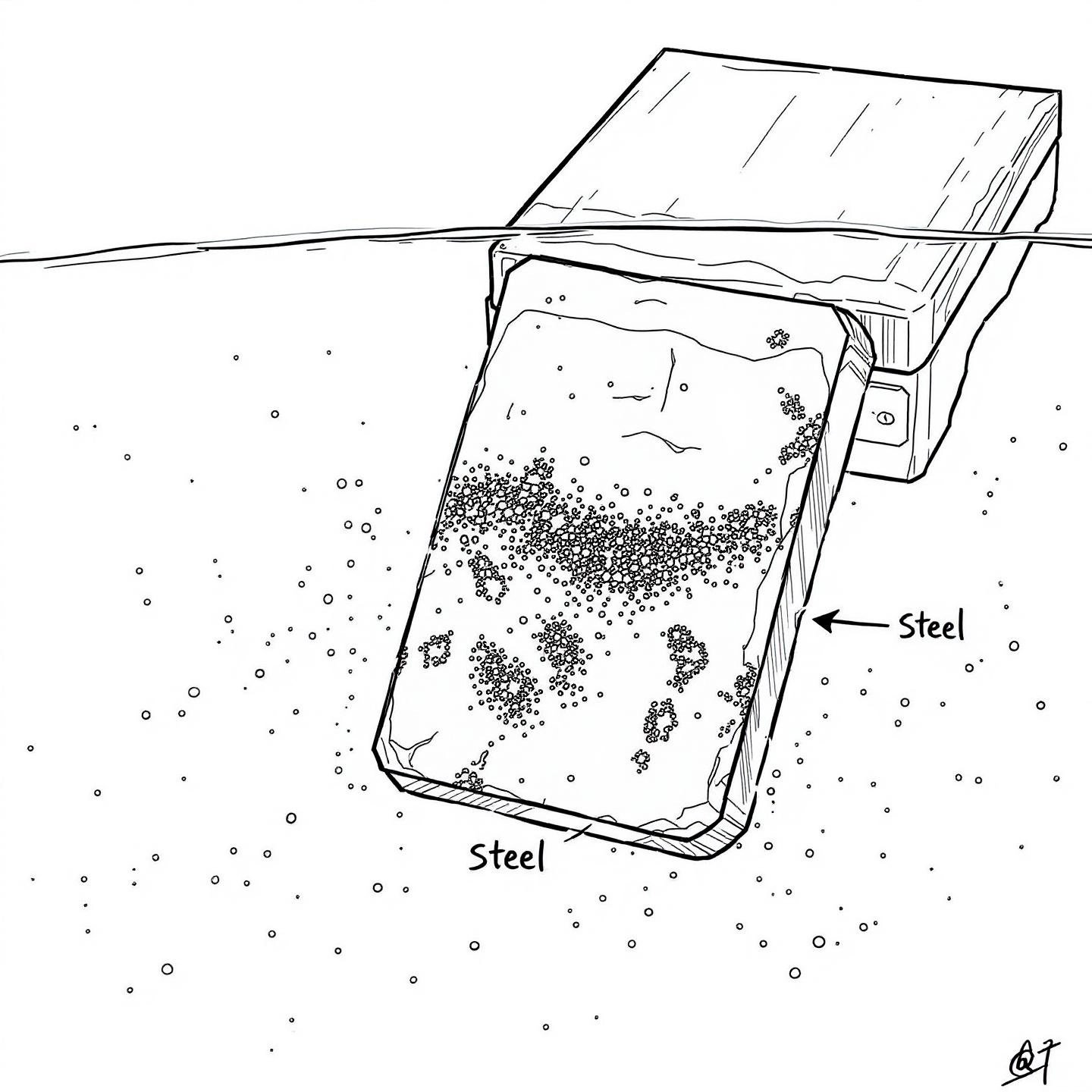
Picture this: you’ve installed a sleek aluminum handrail on your seaside deck, or you’re prepping your boat for a summer on the ocean. You know aluminum doesn’t rust like iron—but can aluminum rust in saltwater or when it touches other metals? The answer is more complex than you might expect, especially in harsh marine or industrial environments.
When aluminum meets saltwater, the main culprit isn’t rust, but pitting corrosion. Saltwater contains chloride ions, which aggressively attack the protective oxide layer that usually shields aluminum. Once this barrier is breached, tiny holes—or "pits"—begin to form on the surface. At first, you might notice a chalky white residue or small, rough spots. Over time, these pits can deepen, compromising both appearance and structural integrity.
Now imagine your aluminum boat has brass or copper fittings, or your building project combines aluminum with steel fasteners. In the presence of water—especially saltwater—this mix can set up an electrochemical reaction called aluminum galvanic corrosion. Here’s how it works:
| Scenario | Corrosion Risk | Prevention Tips |
|---|---|---|
| Aluminum alone in saltwater | Pitting corrosion from chloride attack | Use protective coatings (e.g., powder coating), rinse after exposure |
| Aluminum touching copper/brass in saltwater | Severe galvanic corrosion at contact points | Electrically insulate metals, use compatible fasteners, apply coatings |
| Aluminum with galvanized steel | Lower risk if zinc coating is intact; risk increases if coating fails | Prefer hot-dip galvanized steel, maintain coatings |
Understanding these aggressive corrosion mechanisms is key to making aluminum last in the toughest settings. Next, we’ll look at how these principles apply to everyday items like cans and foil, and what real-world corrosion looks like in your home.
Ever pulled a soda can from the fridge or unrolled a sheet of aluminum foil and wondered, "Do aluminum cans rust?" or "Can aluminum foil rust?" Let’s break down what really happens to these everyday items and why you rarely need to worry about them corroding in normal use.
Aluminum cans are everywhere—from soft drinks to canned foods. Unlike steel cans, which can rust if their protective layer is damaged, aluminum cans don’t contain iron, so they can’t form true rust. But what about corrosion?
Aluminum foil is a staple in kitchens, used for wrapping leftovers, baking, and more. You might notice that even after months in your pantry, foil remains shiny and intact. Here’s why:
In summary, both aluminum cans and foil are engineered—either by coatings or nature—to resist corrosion in everyday life. Unless exposed to extreme conditions or physical damage, these items will last and perform as expected. Next, let’s see how these corrosion principles apply to bigger consumer products, like bikes, cars, and outdoor furniture, and what you can do to keep them looking their best.

Ever left your bike out in the rain or wondered if your aluminum patio set will survive another stormy season? Or maybe you’ve asked, can aluminum bikes rust or can aluminum cars rust? Let’s break down what really happens to aluminum in these common products—and how smart engineering and surface protection keep them looking and performing their best.
Aluminum alloy bicycle frames are a favorite among cyclists for their light weight and impressive durability. Unlike steel, these frames won’t develop orange flakes of rust. Instead, aluminum forms a natural oxide layer that shields the metal from further damage. But does this mean your bike is invincible?
Modern vehicles use aluminum in body panels, frames, and even wheels for its strength-to-weight ratio and corrosion resistance. But what about the question, can aluminum cars rust? The answer: aluminum won’t rust, but it can corrode under certain conditions.
Cast aluminum patio furniture is prized for its weather resistance and stylish designs. Unlike iron or steel, cast aluminum won’t rust, making it a top choice for outdoor living. But does it ever corrode?
Whether you’re a cyclist, car enthusiast, or outdoor entertainer, the right aluminum alloy and surface treatment make all the difference. That’s why industries turn to trusted suppliers like Shengxin Aluminum for high-quality, corrosion-resistant aluminum profiles. With advanced capabilities in anodizing, powder coating, and precision manufacturing, Shengxin delivers components that stand up to the toughest environments—proven in automotive, rail transit, and demanding outdoor applications.
Understanding how and why aluminum products resist corrosion helps you make informed choices and maintain your investments. Next, let’s explore practical steps you can take to further protect your aluminum items from everyday wear and environmental challenges.
Ever wondered how to protect aluminum from rust-like damage or keep your patio furniture, bike, or window frames looking sharp year after year? You’re not alone. While aluminum doesn’t rust, it can corrode—especially in harsh or coastal environments. The good news? With a few simple habits and smart choices, you can dramatically extend the life of your aluminum items and keep them performing their best.
Sounds complicated? Not at all. Here’s a practical checklist to help you prevent aluminum corrosion and safeguard your investment:
| Action | Why It Matters | How To Do It |
|---|---|---|
| Regular Cleaning | Removes dirt, salts, and pollutants that speed up corrosion. |
|
| Apply Protective Coatings | Acts as a barrier against moisture, pollutants, and abrasion. |
|
| Address Scratches and Chips | Prevents corrosive agents from reaching bare metal. |
|
| Prevent Galvanic Corrosion | Stops accelerated corrosion where aluminum contacts other metals. |
|
| Keep Dry When Possible | Limits prolonged moisture that can breach the oxide layer. |
|
By following these straightforward steps, you’ll not only preserve the beauty of your aluminum products but also help them resist the toughest environmental challenges. Next, we’ll wrap up with a summary of key takeaways and why choosing quality aluminum—and protecting it well—matters for the long haul.
When you look at the big picture, the answer to "can aluminum rust" becomes clear: aluminum does not rust like iron, but it does corrode through a unique process. Its natural oxide layer offers a remarkable, self-healing defense—one that sets aluminum apart from many other metals. This protective barrier is the reason why aluminum is trusted in everything from patio furniture and bikes to high-tech transportation and industrial systems.
Imagine your next project—whether it's a modern building facade, rail transit component, or outdoor structure—lasting for decades with minimal upkeep. That's the real promise of expertly treated, durable aluminum profiles. Manufacturers with advanced capabilities, like Shengxin Aluminum, use state-of-the-art extrusion, anodizing, and powder coating to deliver aluminum products that meet the strictest standards for performance and longevity.
Choose a partner with proven expertise and advanced production capabilities. By understanding how aluminum resists corrosion—and by selecting quality materials and finishes—you ensure your projects remain strong, beautiful, and low-maintenance for years to come.
No, aluminum does not rust because it contains no iron. Instead, it forms a protective aluminum oxide layer when exposed to air or moisture. This layer shields the metal from further corrosion, unlike iron, which develops flaky, damaging rust.
To prevent corrosion, keep aluminum surfaces clean and dry, apply protective coatings like anodizing or powder coating, and separate aluminum from dissimilar metals to avoid galvanic corrosion. Regular maintenance and prompt touch-ups on scratches help extend the life of aluminum products.
Aluminum is widely used in marine and coastal applications due to its corrosion resistance. However, saltwater can cause pitting corrosion, especially if the protective oxide layer is damaged. Using anodized or powder-coated aluminum and rinsing with fresh water after salt exposure minimizes risk.
Aluminum cans are lined with protective coatings to prevent corrosion from food and beverages, while aluminum foil forms a stable oxide layer that resists corrosion in typical household use. Unless the coating or foil is damaged, corrosion is rare and usually minor.
Shengxin Aluminum offers advanced surface treatments, including anodizing and powder coating, ensuring high durability and corrosion resistance. Their expertise and large-scale production make them a trusted supplier for demanding industries such as automotive, rail transit, and construction.
 un service en ligne
un service en ligne 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360